Sperm Sexing – New Perspectives in Evaluating Male Reproductive Function
Ramakrishnan Vishwanath and Juan F. Moreno
Research & Development,
Sexing Technologies, Navasota, Texas, USA
Introduction
An estimate of male reproductive function in any of the productive animal species is a topic of varied interpretation. In its simplest form, the aspect of natural mating provides a direct estimate of the fertility potential of a given male. In cattle, in a herd situation, if a male is allowed to naturally service a number of females over a sixty to ninety day period, the fertility potential of the male is the percentage of females successfully fertilised and pregnant. However, this is not easily correlated with the widespread use of this male for artificial breeding. Many other factors come into play such as the quality of the individual ejaculate, the variation between ejaculates, dilution of semen, sperm dose rate per breeding unit, the functional competence of this sperm, the inherent fertility of the females inseminated with this semen, management factors within the herd environment and so on (Amann, 1989, Amann and DeJarnette, 2012).
Conventional semen analysis such as microscopy, CASA analysis and simple viability tests have been used over many years and served its purpose for a high level assessment. As the users have become more discerning and require discrimination of samples across a more narrow range, this type of semen analysis is limited in its application. The fundamental difference in an in vitro laboratory assessment is that a sub set (diluted semen used as frozen straws) of a full breeding unit (whole ejaculate) is used as a measure of male reproductive function. The inherent heterogeneity within a sperm sample and the well-recognized variation between ejaculates of the same animal contribute in no small measure to the variation seen in male reproductive function. There are many reviews on measures of semen competence and in the same vein measures for semen fertility in field situations. Many of them claim varying degrees of correlation between these two measures. Equally, there are also many scholarly articles that explain the disconnect between measures of semen competence and relationship with field fertility. (Evenson et al, 1980, Amann, 1989, Correa et al, 1997, Pentrunkina et al 2007, Gillian et al 2008, Petrunkina and Harrison 2011). When you overlay sperm sexing into this mix, the evaluation of male fertility becomes even more complicated.
Overlaying sperm sexing on male fertility
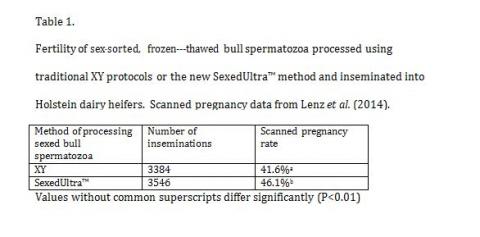
The functional elements of sex sorting and the associated technologies associated with this process have been discussed in many reviews and the references therein (Seidel 2012, Seidel and Garner, 2002, de Graaf et al, 2014). In all these publications the most quoted issue is the relative fertility of sex-sorted sperm compared with unsorted sperm. It has been an axiom that sex sorted semen in cattle has always lagged behind conventional semen in terms of fertility. The compensable elements that normally would lift the sub fertile individual into one with average fertility such as higher sperm numbers or a higher proportion of sperm with better morphological features have not yielded better results with sexed sperm (De Jarnette et al 2011). The economics of this technology and its application in both cattle breeding as well as herd improvement is well understood, yet, the prevailing opinion is that this less than optimum fertility has been the reason why this technology has not been widely adopted (Seidel 2014). This situation is poised for a change. Recent trials with quite significant changes in the overall processing method of sex-sorted semen has for the first time in many years demonstrated a marked increase in fertility (Table-1, Lenz et al 2014)
The difference in fertility between conventional semen and sex-sorted semen is considered to be in the order of 10 percentage points, and this gap is not bridged by increasing the number of sex sorted sperm per inseminate (DeJarnette et al 2011). The causes for this difference in fertility have been attributed to the varied biochemical changes that sperm undergo during the process of sex sorting. The process includes an extended holding time prior to staining, exposure to a laser beam to fluoresce and be discriminated into X and Y sperm and finally exposure to an electrical field for drafting as a pure population into an appropriate vessel, All of this in some part is believed to contribute to this fertility difference (Seidel and Garner 2002).
The challenge therefore has been to seek imaginative ways in both improving sorting techniques with new hardware and software as well as semen processing post sorting (Sharpe and Evans 2009, Evans 2010). New methods developed in semen handling and processing before, during and after sorting in the last few years have now broken this barrier and the fertility gap between conventional semen and sex-sorted semen has reduced (Lenz et al, 2014, deGraf et al 2014). This brings about a new point of discussion. Where exactly is the main lesion that actually causes this drop in fertility with sex-sorted semen?
Sperm Heterogeneity – a factor that affects in vivo fertility of sex sorted semen
It is important to define the term sperm heterogeneity and over the decades it has been recognized with some quite distinct functions. The first is structural heterogeneity between sperm within a sample. As early as 1973, Bedford et al described quite elegantly the variations in human sperm nuclear chromatin assembly with some distinct pattern and arrangements (Bedford, 1973). Similar such observations were seen in bull sperm with a comment on association of such variation with fertility (Ballachey et al 1987).
The second is functional heterogeneity and this has been explained by classical competition studies where adaptation, sexual selection and choice of mate due to fitness traits is done through physiological means by the female reproductive tract (Curtsinger, 1991, Birkhead and Moller, 1993, Birkhead and Pizzari, 2002). Several studies have tried to rationalize these concepts through some innovative experiments such as heterospermic inseminations (Beatty et al 1969). These concepts have been described in more detail with an accompanying thesis on how to exploit this heterogeneity to develop rational laboratory tests for sperm competence (Holt and van Look, 2004).
The third concept is physiological heterogeneity between populations of sperm within an ejaculate. The simple explanation for this is where a semen sample has distinct sub populations that would physiologically be ready for fertilisation at different times post insemination. This will allow some flexibility from the time the sperm enter the female reproductive tract to the time when ovulation occurs and a competent sub set of sperm are available and ready for fertilization. The variation in fertility of a given semen sample or of semen samples from the same individual is attributed to this diversity in a sperm population within an ejaculate (Rodriguez- Martinez 2006). If this heterogeneity in sperm population is disturbed, it is more than likely to lead to sub fertility or infertility. A good illustration of this concept can be seen in trials with fresh encapsulated sperm compared with fresh conventional sperm (McMillan and Vishwanath 1994, Vishwanath et al 1997).
Parallels between encapsulated sperm and sex sorted sperm
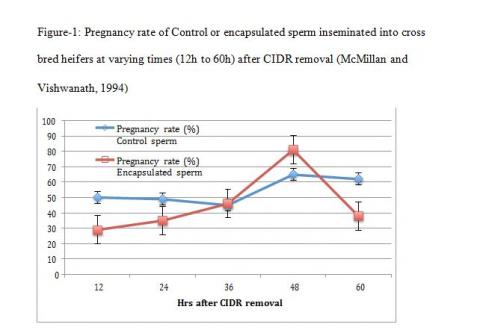
The theory behind encapsulation was to be able to encase sperm in an inert environment shielded from the uterine milieu after insemination. This would provide a mechanism for discrete packets of sperm to be released at different times as the capsules disintegrate and, therefore, being available for fertilization over extended periods in the female reproductive tract (Nebel et al 1993, 1995, Vishwanath et al 1997). However, nature decreed otherwise and the outcome was quite different. When encapsulated sperm and conventional sperm were inseminated at optimum times, fertility between the two was comparable with encapsulated sperm in a minor way outperforming conventional sperm (Vishwanath et al 1997). However, when the system was stretched and when inseminations were conducted across the spectrum from pro-oestrus to post-oestrus, the result was quite the contrary to what would be expected of a slow release system to accommodate this stretch. Conventional unfrozen semen retained high fertility over an extended period on either side of an ovulatory response, however, encapsulated semen was highly fertile in a very narrow window and on either side of the optimal period was markedly sub fertile (Figure – 1, McMillan and Vishwanath 1994 ). A similar parallel is seen with sex sorted semen as well. In a recent study in beef cattle fertility of sex sorted semen was comparable to conventional semen when inseminated almost 20 hrs after the recommended time for insemination (Thomas et al 2014). The conclusion we can draw from this observation is that somehow during the sperm sorting and freezing process, the heterogeneity of these sperm cells is interminably altered and they are remarkably fertile in a very narrow window.
Sexed sperm – effect of sperm numbers and cryopreservation
The relationship between sperm number and the fertility of a given semen sample is well understood. Studies by Pace et al 1981 and Den Daas et al 1998 showed that increasing sperm numbers increased fertility until it reached the asymptotic maximum for the given bull. While individual bulls varied in their absolute fertility once they reached their asymptote, increasing sperm numbers did not alter this maximum. It is reasonable to assume that this concept would hold true with sex-sorted sperm as well. This theory was tested in one study where comparable numbers of sex-sorted and control sperm still showed decreased fertility (Frijters et al 2009) and in a separate study where increasing the number of sex sorted sperm from 2.1 million to 3.5 million per inseminate also did not improve fertility compared with control non sorted sperm (De Jarnette et al 2011). This demonstrates that sex sorted sperm are physiologically different compared with unsorted sperm and the usual compensable elements such as increasing sperm numbers improving in vivo fertility does not apply in this case. However, it is important to note that sex sorted sperm has never been tested at extremely high concentrations such as 10 to 25 million per inseminate. It is neither practical nor economical to do so given the constraints of the sex sorting process.
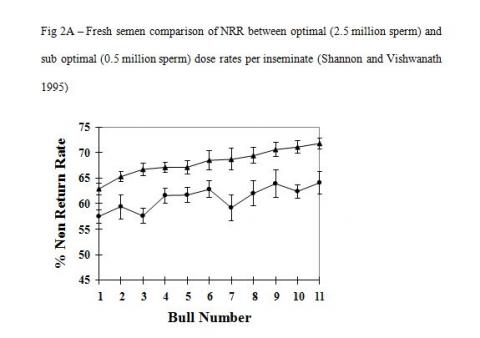
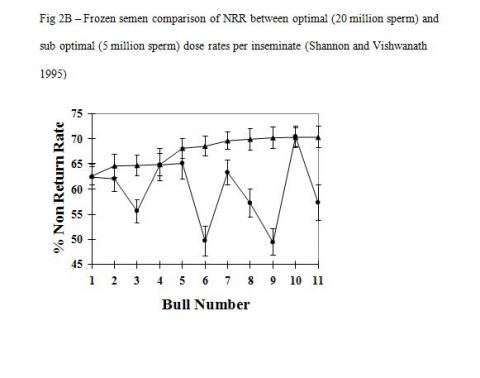
There is good evidence that cryopreservation in itself causes irreparable damage to the inherent fertility potential of conventional sperm. The important factor here is that the heterogeneous sperm populations in conventional semen samples react differently to the cryopreservation process and hence the detrimental effect of cryopreservation is countered by increasing sperm numbers. Studies with optimal and sub optimal numbers of sperm used as unfrozen or cryopreserved semen show vastly different responses in overall fertility (Figure-2A & B, Shannon and Vishwanath 1995). A five-fold increase in sperm numbers is required to fully compensate for cryopreserved damage compared with unfrozen semen.
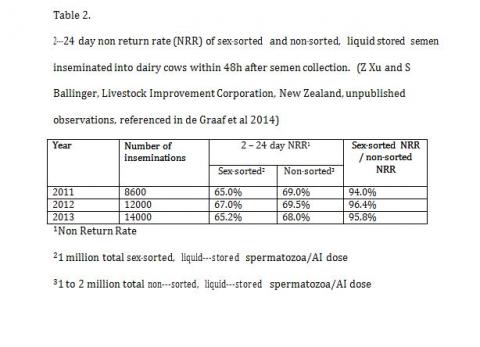
The possibility that the interaction between the process of sex sorting and subsequent cryopreservation is multiplicative and requires a lot more sperm to fully reach its full potential has been mooted by George Seidel (2012). This is in fact true and there is growing evidence that the actual process of sex sorting itself is not quite as damaging and fertility with equal numbers of fresh sex sorted or unsorted semen is comparable in fertility. Large scale fertility trials in New Zealand with sex sorted and fresh control semen provide ample evidence that the sex sorting process per se is not detrimental to overall fertility but the interaction of sex sorting with cryopreservation is far more damaging. (Table-2).
Testing the heterogeneity theory with sex sorted sperm
The evidence is mounting that the sex sorting process combined with the freezing process alters the heterogeneity of the sperm population. This effect is not so apparent in fresh sex sorted semen. Some imaginative trials now need to be conducted with both fresh and frozen sex sorted sperm across either side of ovulation from pro-oestrus to post-oestrus animals to dissect out this response
Conclusion
Improvements in semen technology have now contributed to a marked lift in fertility with sex sorted frozen semen. It is abundantly clear that there is no perceptible loss in fertility with fresh sex sorted sperm and further improvements in fertility with sex sorted frozen sperm is possible with better freezing technologies and timing of insemination relative to oestrus.
References
Amann RP. Can the fertility potential of a seminal sample be predicted accurately? J. Androl. 1989; 10, 89 - 98.
Amann RP. and DeJarnette JM. Impact of genomic selection of AI dairy sires on their likely utilization and methods to estimate fertility. A paradigm shift. Therio. 2012; 77, 795 – 817
Ballachey, BE, Hohenboken, WD, Evenson, DP. Heterogeneity of sperm nuclear chromatin structures. Biol. Reprod. 1987; 36, 915 – 925
Bedford, JM. Components of sperm maturation in the human epididymis. Advances in the biosciences, 1973, Vol.10, pp.145-55.
Beatty RA, Bennett GH, Hall JG, Hancock JL, Stewart DL. An experiment with heterospermic insemination in cattle. J. Reprod. Fert. 1969, 19: 491 – 502. Birkhead TR, Moller AP. Sexual selection and the temporal separation of reproductive events: sperm storage data from reptiles, birds and mammals. Biol. J. Linn. Soc. 1993, 50: 295 – 311.
Birkhead TR, Pizzari T. Postcopulatory sexual selection. Nat. Rev. Genet. 2002, 3: 262 – 273.
Curtsinger, JW. Sperm competition and the evolution of multiple mating. American
Naturalist 1991, 138: 93 - 102
Correa JR, Pace MM, Zavos PM. Relationships among frozen-thawed sperm characteristics assessed via the routine semen analysis, sperm functional tests and fertility of bulls in an artificial insemination program. Therio. 1997; 48: 721 – 731. den Daas JHG, de Jong G, Lansbergen LMTE, van Wagtendonk-de Leeuw AM. The relationship between the number of spermatozoa inseminated and the reproductive efficiency of individual dairy bulls. J. Dairy Sci. 1998, 81: 1714–23
DeGraaf SP, Leahy T, Vishwanath R. Biological and practical lessons associated with the use of sexed semen. Rumin. Reprod. Symp. 2014. In press
DeJarnette JM, Leach MA, Nebel RL, Marshall CE, Cleary CR, Moreno JM. Effects of sex sorting and sperm dosage on conception rates of Holstein heifers; is comparable fertility of sex sorted and conventional semen plausible? J. Dairy Sci. 2011; 3477-3483
Evans KM. Interpretation of sex sorting process and new developments. Proc. 23rd Tech. Conf. Artif. Insem. Reprod. National Association of Animal Breeders. 2010; Pp 93-98
Evenson DP, Darzynkiewicz Z, Melamed MR. Relation of mammalian sperm chromatin heterogeneity to fertility. Science 1980; 210: 1131-1133
Frijters ACJ, Mullaart E, Roelofs RGM, van Hoorne RP, Moreno JF, Moreno D, Merton JS. What affects fertility of sexed bull semen more, low sperm dosage or the sorting process? Theriogenology 2009; 71:64-67.
Gillan l, Kroetsch T, Maxwell WM, Evans G. Assessment of in vitro sperm characteristics in relation to fertility in dairy bulls. Anim Reprod. Sci. 2008; 103. 201 – 214.
Holt WV, Van Look KJW. Concepts in sperm heterogeneity, sperm selection and sperm competition as biological foundations for laboratory tests of semen quality. Reprod. 2004, 127: 527 – 535.
Lenz RW, Gilligan TB, DeJarnette JM, Utt MD, Hesler LA, Evans KM, Gonzalez- Marin C, Moreno JF, Vishwanath R. SexedUltra™ a new method of processing sex- sorted bovine sperm improves post thaw semen quality and conception rate. Submitted for Publication Therio. 2014
McMillan WH, Vishwanath R, Pregnancy rate in heifers inseminated with control or encapsulated sperm prior to, during or after onset of oestrus. Proc. VIIth Intl. Symp. Spermatology. Cairns, Australia. 1994. Abstr No: 9.25
Nebel RL, Vishwanath R, McMillan WH, Saacke RG. Microencapsulation of bovine spermatozoa for use in artificial insemination: a review. Reprod Fertil Dev. 1993,
5(6):701-12.
Nebel RL, Vishwanath R, Pitt CJ, Mcmillan WH, Saacke RG. Microencapsulation of sperm for use in artificial insemination of the bovine. In “Reproduction and Animal Breeding, Advances and Strategy” ed: G. Enne, G.F. Greppi and A. Lauria, Elsevier Science Publishers BV, (1995). pp 89-97.
Pace MM, Sullivan JJ, Elliott FI, Graham EF and Coulter GH. Effects of thawing temperature, number of spermatozoa, and spermatozoal quality on fertilizing capacity of bovine spermatozoa packaged in 0.5ml-french straws. J. Animal Science, 1981. 53[3]: 693-70
Petrunkina AM, Harrison RAP. Cytometric solutions in veterinary andrology: Developments, advantages and limitations. Cytometry 2011; 79A. 338 - 348
Petrunkina AM, Waberski D, Gunzel-Apel AR, Topfer-Petersen E. Determinants of sperm quality and fertility in domestic species. Reproduction 2007; 134: 3 – 17. Rodriguez-Martinez H. Can we increase the estimative value of semen assessment. Reprod. Dom. Anim 2006, 41: Suppl 2, 2 – 10.
Seidel Jr GE. Sexing mammalian sperm where do we go from here? J Reprod Dev. 2012; 58(5):505-9. Review.
Seidel Jr GE. Update on sexed semen technology in cattle. Animal 2014, 8 (1): 160 - 164
Seidel Jr GE, Garner DL. Current status of sexing mammalian spermatozoa. Reproduction 2002;124. 733–743.
Shannon P, Vishwanath R. The effect of optimal and sub optimal concentrations of sperm on the fertility of fresh and frozen bovine semen. Anim. Reprod. Sci. 1995, 39: 1-10.
Sharpe JC and Evans KM. Advances in flow cytometry for sperm sexing. Theriogenology. 2009; 71. 4-10.
Thomas JM, Lock SL, Poock SE, Ellersieck MR, Smith MF Patterson DJ. Delayed insemination of non-estrus cows improves pregnancy rates when using sex-sorted semen in timed artificial insemination of suckled beef cows. J. Anim. Sci. 2014, 92: 1747 – 1752.
Vishwanath R, Nebel RL, McMillan WH, Pitt CJ, Macmillan KL. Selected times of insemination with microencapsulated bovine spermatozoa affect pregnancy rates of synchronized heifers. Therio. 1997, 48: 369 – 376.
Vishwanath, R., Shannon, P., Do sperm cells age? A review of the physiological changes in sperm during storage at ambient temperatures. Reprod. Fertil. Dev. 1997: 9. 321–331.
Dr. Ramakrishnan Vishwanath
Education:
Obtained his Bachelors and Master’s degree with distinction from Osmania University, Hyderabad. He received Ph.D from University of Sydney in 1989.
Current Professional Engagement:
Dr. Vishwanath is presently Director, Global Research and Development, Sexing Technologies, USA and Managing Director, Vintara consultants, New Zealand. He is also Director in 2 more organisations in New Zealand. As an adjunct faculty, he is associated with Texas A&M University, USA.
Professional Experiences:
Extensive experience in Research and Development with specialist knowledge in the artificial breeding industry in dairy cattle, reproductive biotechnologies, semen biochemistry, product development, project management and staff management. Excellent track record as a world acknowledged specialist in reproductive technologies with over 50 publications, guiding Masters and Ph.D scholars. Credited with 7 patents and patent applications related to compositions and methods for improving the quality of processed sperm, bulk freezing of semen, Methods of processing sperm for sex sorting etc.
Contact: vish@sexingtechnologies.com